 Polymer Properties Database
Polymer Properties Database
Polyphosphazenes
Properties
Polyphosphazenes are by far the largest class of inorganic polymers. Hundreds of different polymers of this type have been synthesized, with a range of physical and chemical properties that rival those of organic polymers.1 This class of polymers possesses many valued properties such as high thermal and chemical stability and comparatively low toxicity which makes them attractive materials for numerous biomedical, elastomeric, and electrochemical applications. The high thermal-oxidative stability of the polymer backbone has been explained by the high oxidation state of the main chain atoms which means that the thermodynamic driving force for oxidative skeletal cleavage is rather weak. Most of the other properties depend to a large extent on the type of substituents on the polymer backbone and on the chain architecture including glass-transition and melting temperatures, modulus and tensile strength as well as hydrolytic stability, solubility in solvents and compatibility with other polymers. These properties can be varied over a wide range by changing the type of side groups, the molecular weight, and cross-link density.
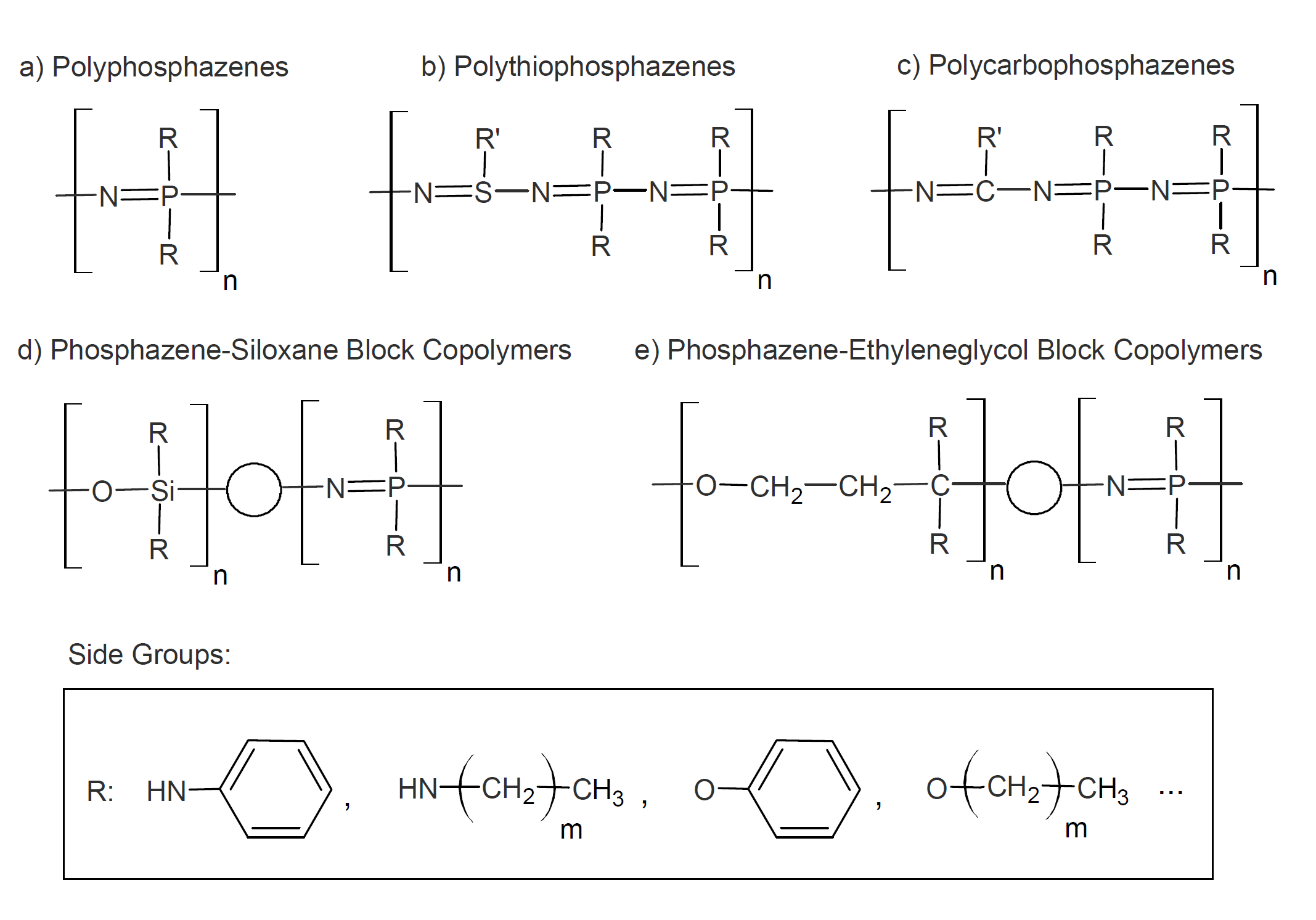
Some forms of unmodified and modified polyphosphazenes are depicted below. The polymer backbone of unmodified polyphosphazene consists of alternating phosphorus and nitrogen atoms, with two side groups, R, being attached to each phosphorus (a). These side groups may be organic, organometallic, or inorganic. Also obtainable are polymers in which a portion of the phosphorus atoms has been replaced by sulfur (b) or carbon atoms (c) or in which polyphosphazenes are linked to other polymers such as polysiloxanes (d) or polyethers (e).
Synthesis
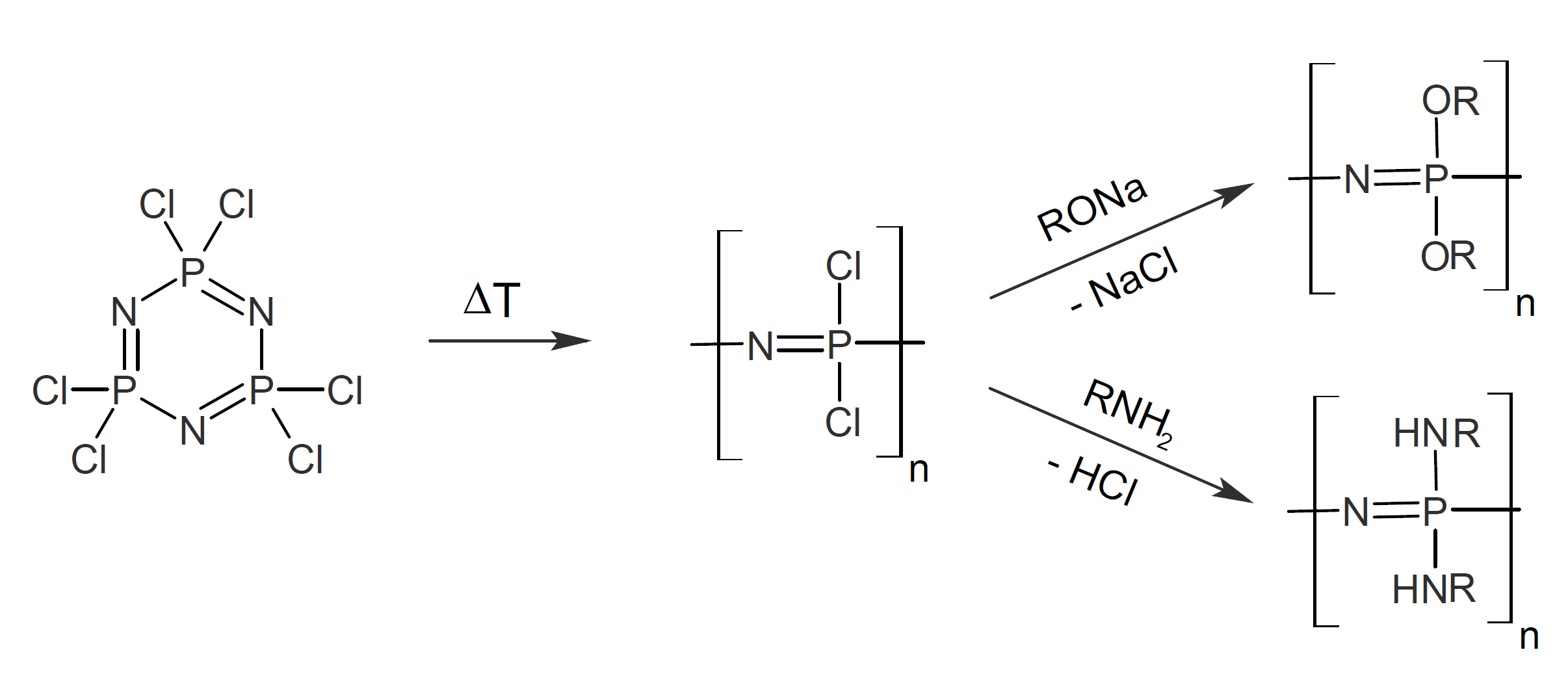
Polyphosphazenes are produced by a unique synthesis method that allows the types of side groups, R, to be varied over a very broad range. The most common method uses hexachlorocyclotriphosphazene (phosphonitrilic chloride trimer) as a starting material. It is first converted to poly(dichlorophosphazene) by ring-opening polymerization at elevated temperature (250°C) which yields polymers of high molecular weight (106 - 107 g/mol). The resulting linear polymers can be dissolved completely in organic solvents such as benzene, toluene, or tetrahydrofuran.1 Subsequent reaction of the dissolved linear poly(dichlorophosphazene) with sodium alkoxides or alkylene amines yields polyalkoxyphosphazenes and polyaminophosphazenes, respectively, whereas further heating of poly(dichlorophosphazene) causes cross-linking of the chains and yields insoluble inorganic elastomers.
Besides linear polyphosphazenes, other molecular architectures such as block copolymers, comb copolymers, cyclo-linear polymers, and star polymers have also been synthesized. Particularly noteworthy are phosphazene-organic and phosphazene-polysiloxane block copolymers, copolymers with six-membered phosphazene rings in the polymer backbone, as well as comb copolymers with (short) phosphazene or organic branches linked to phosphorus atoms in the main chain.1
Commercial MF Resin Products
Major manufacturers of polyphosphazenes are White Square Chemical, Neo-Advent Technologies, and CM-Tecs. They are marketed under the trade names PNF™ and NOVUS®.
Applications
Polyphosphazenes can be used in very demanding engineering applications. For example, elastomeric (fluorinated) polyphosphazenes can be employed as solvent- and heat resistant seals, O-rings and gaskets and foamed products as fire- and heat resistant sound-insulation materials.4 However, the production of elastomeric and foamed polyphosphazenes was discontinued because the manufacturing process was too expensive.
An important area where polyphosphazenes excel is in the field of of implantable medical devices and pharmaceutical products. These polymers have been proven to be biocompatible, biodegradable, and bioactive,3 and can be tailored to be biostable or biodegradable. Major (potential) applications include biodegradable release and encapsulation materials in drug delivery systems; scaffolds for tissue engineering; biological separation membranes for air enrichment, electrodialysis, and water/gas purification; as well as medical components such as prosthetics, denture liners, and implants.